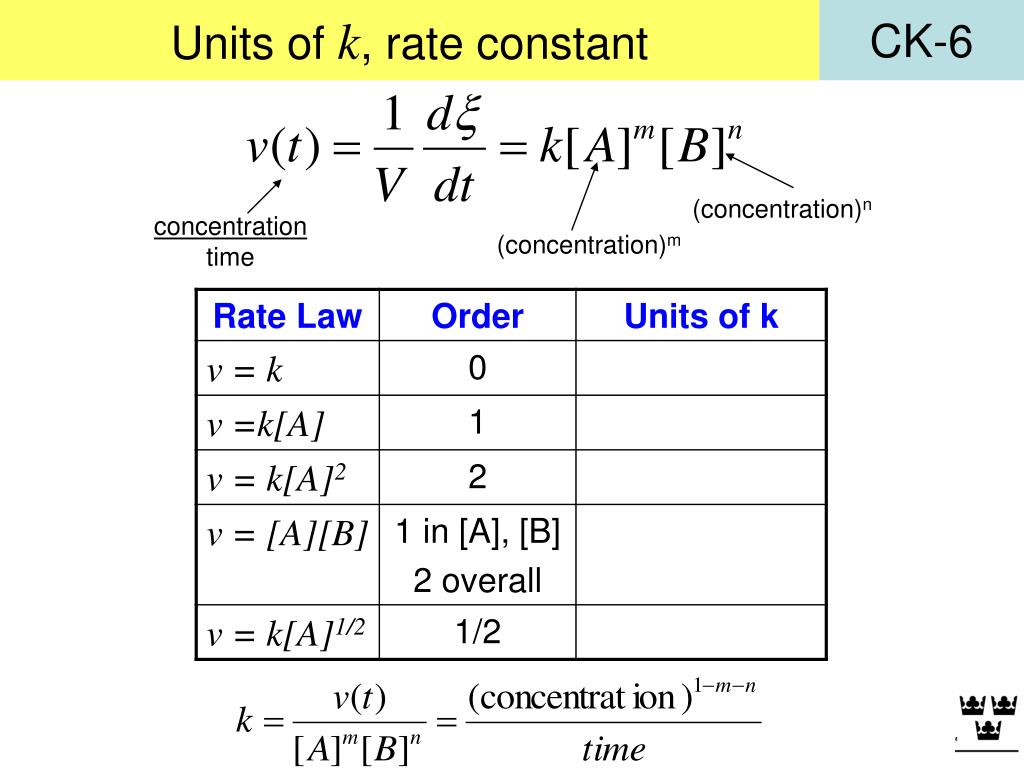
Rate Constant K Has Units . Determine the numerical value of the rate constant k with appropriate units. Experimental data show that k has the value 5.15 × 10 −4 s −1 at 25°c. A very small value for the rate constant equates to a very slow reaction in. The rate constant, k, gives a direct measure of the relative reaction rate. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant has units of reciprocal seconds (s −1) because the. The units for the rate of a reaction are mol/l/s. Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate constant k will depend on the exponents of the.
from www.slideserve.com
The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant has units of reciprocal seconds (s −1) because the. Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate constant k will depend on the exponents of the. Experimental data show that k has the value 5.15 × 10 −4 s −1 at 25°c. The units for the rate of a reaction are mol/l/s. A very small value for the rate constant equates to a very slow reaction in. Determine the numerical value of the rate constant k with appropriate units. The rate constant, k, gives a direct measure of the relative reaction rate.
PPT Chemical PowerPoint Presentation, free download ID315859
Rate Constant K Has Units Determine the numerical value of the rate constant k with appropriate units. Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate constant k will depend on the exponents of the. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant has units of reciprocal seconds (s −1) because the. The units for the rate of a reaction are mol/l/s. The rate constant, k, gives a direct measure of the relative reaction rate. Experimental data show that k has the value 5.15 × 10 −4 s −1 at 25°c. Determine the numerical value of the rate constant k with appropriate units. A very small value for the rate constant equates to a very slow reaction in.
From www.youtube.com
Units of rate constant calculate rate constant units units of first Rate Constant K Has Units Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate constant k will depend on the exponents of the. Determine the numerical value of the rate constant k with appropriate units. The units for the rate of a reaction are mol/l/s. The rate constant has units of reciprocal seconds (s −1) because the. Experimental. Rate Constant K Has Units.
From www.slideshare.net
Chapter 14 Lecture Chemical Rate Constant K Has Units Experimental data show that k has the value 5.15 × 10 −4 s −1 at 25°c. Determine the numerical value of the rate constant k with appropriate units. A very small value for the rate constant equates to a very slow reaction in. The units for the rate of a reaction are mol/l/s. The rate constant, k, gives a direct. Rate Constant K Has Units.
From www.doubtnut.com
Plots showing the variation of the rate constant (k) with temperature Rate Constant K Has Units A very small value for the rate constant equates to a very slow reaction in. The units for the rate of a reaction are mol/l/s. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Experimental data show that k has the value 5.15 ×. Rate Constant K Has Units.
From www.youtube.com
Units of the rate constant AP Chemistry Khan Academy Rate Constant K Has Units Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate constant k will depend on the exponents of the. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The units for the rate of a reaction are mol/l/s.. Rate Constant K Has Units.
From www.youtube.com
Units of Rate Constant (k) YouTube Rate Constant K Has Units The rate constant, k, gives a direct measure of the relative reaction rate. The units for the rate of a reaction are mol/l/s. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Determine the numerical value of the rate constant k with appropriate units.. Rate Constant K Has Units.
From www.chegg.com
Solved Determine the units on the rate constant, k, for a Rate Constant K Has Units The units for the rate of a reaction are mol/l/s. Determine the numerical value of the rate constant k with appropriate units. The rate constant, k, gives a direct measure of the relative reaction rate. A very small value for the rate constant equates to a very slow reaction in. The rate constant has units of reciprocal seconds (s −1). Rate Constant K Has Units.
From www.chegg.com
Solved What is the value of the rate constant k for this Rate Constant K Has Units The units for the rate of a reaction are mol/l/s. A very small value for the rate constant equates to a very slow reaction in. The rate constant, k, gives a direct measure of the relative reaction rate. Experimental data show that k has the value 5.15 × 10 −4 s −1 at 25°c. Since the rate of a reaction. Rate Constant K Has Units.
From www.chegg.com
Solved Calculate the value of the true rate constant, k, Rate Constant K Has Units The rate constant, k, gives a direct measure of the relative reaction rate. Determine the numerical value of the rate constant k with appropriate units. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The units for the rate of a reaction are mol/l/s.. Rate Constant K Has Units.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial Rate Constant K Has Units The units for the rate of a reaction are mol/l/s. The rate constant, k, gives a direct measure of the relative reaction rate. Determine the numerical value of the rate constant k with appropriate units. A very small value for the rate constant equates to a very slow reaction in. The rate constant has units of reciprocal seconds (s −1). Rate Constant K Has Units.
From mckinleytrust.blogspot.com
Unit of Rate Constant for Third Order Reaction Mckinleytrust Rate Constant K Has Units A very small value for the rate constant equates to a very slow reaction in. Experimental data show that k has the value 5.15 × 10 −4 s −1 at 25°c. The units for the rate of a reaction are mol/l/s. Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate constant k will. Rate Constant K Has Units.
From www.slideserve.com
PPT Chemistry 102(01) Spring 2012 PowerPoint Presentation, free Rate Constant K Has Units Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate constant k will depend on the exponents of the. The rate constant, k, gives a direct measure of the relative reaction rate. The rate constant has units of reciprocal seconds (s −1) because the. Experimental data show that k has the value 5.15 ×. Rate Constant K Has Units.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID315859 Rate Constant K Has Units The rate constant has units of reciprocal seconds (s −1) because the. Experimental data show that k has the value 5.15 × 10 −4 s −1 at 25°c. Determine the numerical value of the rate constant k with appropriate units. Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate constant k will depend. Rate Constant K Has Units.
From www.youtube.com
The Rate Constant k YouTube Rate Constant K Has Units The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant, k, gives a direct measure of the relative reaction rate. Experimental data show that k has the value 5.15 × 10 −4 s −1 at 25°c. The units for the rate of. Rate Constant K Has Units.
From www.homeworklib.com
Review Constants Periodic T Part A The rate constant for a certain Rate Constant K Has Units The units for the rate of a reaction are mol/l/s. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Since the rate of a reaction has the dimensions of (concentration/time), the dimensions of the rate constant k will depend on the exponents of the.. Rate Constant K Has Units.
From www.slideshare.net
Chemical Rate Constant K Has Units The rate constant has units of reciprocal seconds (s −1) because the. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Experimental data show that k has the value 5.15 × 10 −4 s −1 at 25°c. A very small value for the rate. Rate Constant K Has Units.
From saylordotorg.github.io
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Orders Rate Constant K Has Units Determine the numerical value of the rate constant k with appropriate units. The units for the rate of a reaction are mol/l/s. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant has units of reciprocal seconds (s −1) because the. Since. Rate Constant K Has Units.
From www.youtube.com
The rate constant `(K\')` of one reaction is double of the rate Rate Constant K Has Units The rate constant has units of reciprocal seconds (s −1) because the. Determine the numerical value of the rate constant k with appropriate units. A very small value for the rate constant equates to a very slow reaction in. The rate constant, k, gives a direct measure of the relative reaction rate. Experimental data show that k has the value. Rate Constant K Has Units.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID2054453 Rate Constant K Has Units The units for the rate of a reaction are mol/l/s. The rate constant has units of reciprocal seconds (s −1) because the. Experimental data show that k has the value 5.15 × 10 −4 s −1 at 25°c. A very small value for the rate constant equates to a very slow reaction in. Since the rate of a reaction has. Rate Constant K Has Units.